Panbio Covid 19 Igg Igm Rapid Test Device Abbott
Abbott has launched its third test for coronavirus covid 19 and is shipping tests to hospitals across the u s.
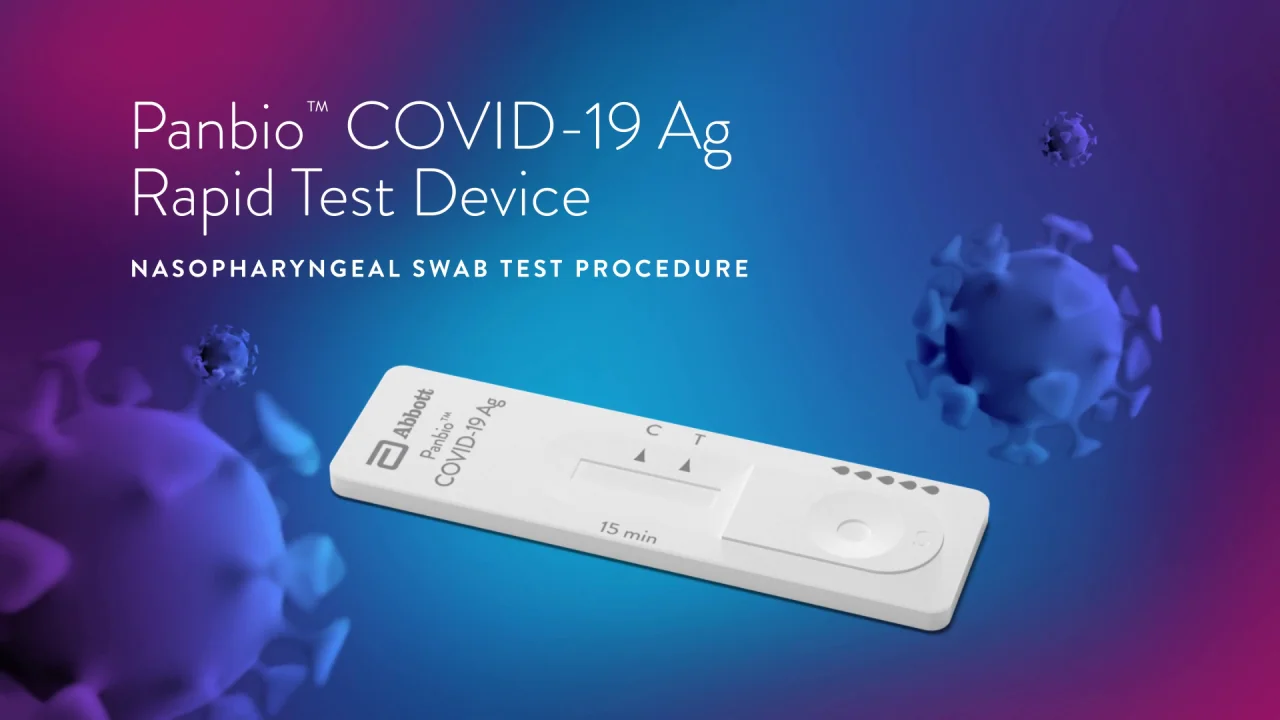
Panbio covid 19 igg igm rapid test device abbott. Detecting these igg antibodies will help determine if a person was previously infected with the virus that causes covid 19. Abbott launched its third covid 19 test and will start shipping in the u s. The abbott panbio covid 19 igg igm rapid test device panbio is ideally suited to meet this unmet global need because it is a rapid antibody detection lateral flow test that is simple to use requires a small drop of whole blood or serum and produces a result in 10 20 min. Here are the details.
Serum or plasma should be separated as soon as possible to avoid hemolysis. The covid 19 igm igg antibody rapid test is a fast and effective method for screening igm and igg antibodies against sars cov 2. As new antibody tests become available it is important to evaluate their performance and utility. Conditions have been imposed on the supply of covid 19 serology based and rapid antigen point of care tests.
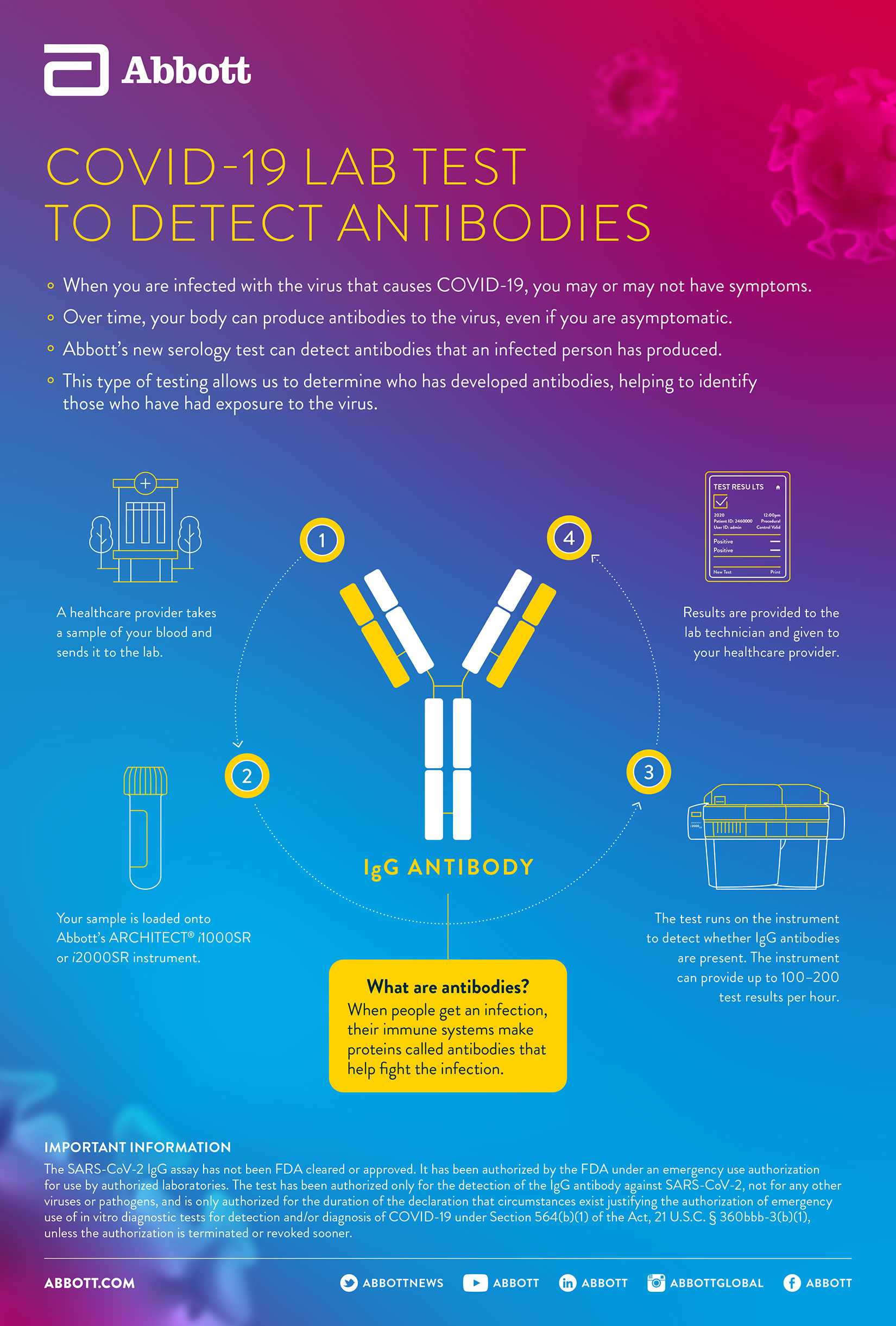
Abbott s test helps to detect the igg antibody to sars cov 2. The peter doherty institute for infection and immunity the doherty institute has been engaged by the department of health to assist with the post market validation of new covid 19 rapid tests to. The test is a serology test also called an antibody test which could be a critical next step in battling this virus. This test can also suggest information on the stage of infection.
The product may be used in any laboratory and non laboratory environment that meets the requirements specified in the. Scale up covid 19 antibody testing. The covid 19 igg igm rapid test device is intended for use with human whole blood serum or plasma specimens only. Intended to be used as an aid in the diagnosis of sars cov 2 infection.
Only clear non hemolyzed specimens are recommended for use with this test. The id now covid 19 assay is now available for use on the id now platform under u s. The panbio covid 19 igg igm rapid test device fingerstick whole. The id now covid 19 rapid test delivers high quality molecular positive results in as little as 5 minutes targeting the coronavirus covid 19 rdrp gene.
Legal supply of covid 19 test kits. Both immunoglobulin m igm and immunoglobulin g igg antibodies are produced during the primary immune response. Food and drug administration emergency use authorization eua. An in vitro diagnostic rapid test for the qualitative detection of igg and igm antibodies to sars cov 2 in human serum plasma venous and fingerstick whole blood.
This new test is a serology test also called an antibody test and helps to detect the igg antibody to sars cov 2.